Abstract
Background: Clofarabine (CLO) is an effective anti-leukemic drug that was recently evaluated in first line treatment of adult patients with acute lymphoblastic leukemia (ALL) in a prospective randomized study (HOVON 100). While more patients achieved eradication of measurable residual disease (MRD), no beneficial effect on the primary endpoint event-free survival (EFS) was noted (Rijneveld et al, 2022). To study these observations in more detail, we applied multi-state modeling to evaluate whether CLO affected intermediate events, including going to off protocol treatment (not due to relapse, but because of treatment-related toxicity) and achieving MRD negativity (defined as MRD<10-4), and the subsequent impact of these events on death or relapse.
Methods: The HOVON 100 study randomized 334 adult ALL patients to the control (n=166) and to the CLO arm (n=168). Parameters determining disease risk were evenly distributed between the two study arms. Patients received induction and consolidation chemotherapy (Ind & Cons) followed by either allogeneic hematopoietic cell transplantation (alloHCT) or maintenance treatment, depending on risk status (irrespective of MRD), donor availability, and transplant eligibility. As recently reported, 5-year EFS was 50% (95%CI 42-57) in control patients compared with 53% (95%CI 45-60) in CLO treated patients. We developed a Markov time-inhomogeneous multi-state model describing the events between Ind & Cons (start state) until the date of last contact, incorporating maintenance treatment, off-protocol treatment, alloHCT, and relapse as intermediate events. Relapse mortality and non-relapse mortality were considered end states. We developed a second Markov time-inhomogeneous multi-state model that incorporated MRD negativity after consolidation as intermediate event, without considering off-protocol treatment. Patients with missing MRD data after consolidation (40%) were not considered MRD negative. All other states were evaluated as mentioned above. Effect estimation was done using a transition-specific Cox model including treatment arm (CLO vs control).
Results: Overall, CLO treated patients went to off-protocol treatment almost as frequent as control patients (45/168 [27%] vs 36/166 [22%], P=0.31), but went to off-protocol treatment more frequently during maintenance treatment (13/44 (30%) vs. 6/56 (11%); HR 2.85 [1.08-7.50], P=0.035). Considering the treatment effect on the transitions to relapse, CLO treated patients had non-significantly different relapse during maintenance (HR 0.58 [0.24-1.39], P=0.22) than control patients. Such an effect was also not observed in alloHCT recipients (HR alloHCT to relapse: 0.86 [0.38-1.92], P=0.71) and was also not observed in patients who went to off-protocol treatment (HR 0.78 [0.31-1.98], P=0.60).
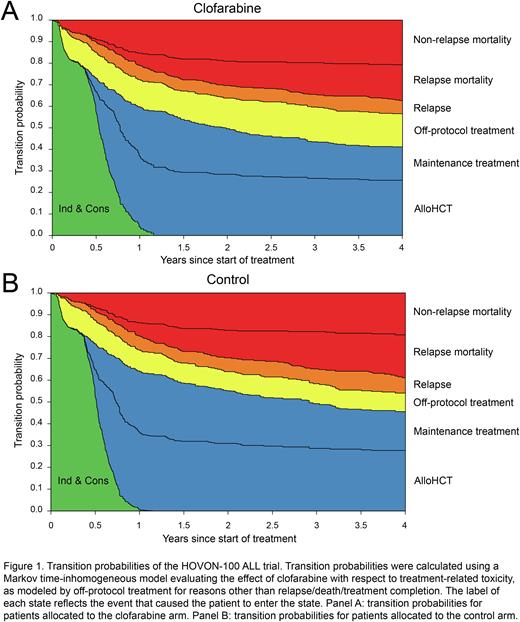
With respect to MRD, patients in the CLO arm had a trend towards increased MRD negativity compared with control patients (HR Ind & Cons to MRD negativity: 1.36 [0.96-1.93], P=0.083). Additionally, MRD negative patients in the CLO arm were less likely to proceed to maintenance treatment (HR 0.45 [0.26-0.80], P=0.0065), did not proceed more often to alloHCT (HR 0.83 [0.50-1.38], P=0.46), and did not show less relapse than patients in the control arm (HR 1.08 [0.27-4.44], P=0.90). Transition probabilities of the multi-state "off-protocol” model per study arm are summarized in Figure 1.
Conclusion: Multi-state modeling, taking going to off-protocol treatment and MRD negativity as intermediate events into account, identified that patients receiving CLO more often went to off-protocol treatment during maintenance, and that CLO treated patients less frequently proceeded to consolidation by alloHCT or maintenance treatment according to protocol. Despite a higher proportion of MRD negativity in patients receiving CLO, not completing protocol treatment might also explain similar relapse rates and EFS in the two arms. The addition of CLO to an already intensified chemotherapeutic schedule resulted in going to off-protocol treatment due to cumulative toxicities and not due to relapse for a higher proportion of patients. Future studies may evaluate CLO in a less intensified induction/consolidation/maintenance chemotherapeutic scheme, which differs from our pediatric inspired, intensified, backbone of the HOVON 100 ALL study.
Disclosures
Biemond:BMS: Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; GBT: Research Funding; Chiesi: Membership on an entity's Board of Directors or advisory committees; Modus Therapeutics: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Chiesi: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; GBT: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanquin: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. van de Loosdrecht:Alexion: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. van der Velden:Cytognos: Other: laboratory services agreement ; Agilent: Other: laboratory services agreement ; Euroflow: Patents & Royalties; BD biosciences: Other: laboratory services agreement .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal